Printing challenges related to medical device inks
Medical device inks serve a variety of purposes. Some are simply related to branding, such as product names or model numbers. Others are used for quality control, such as batch numbers or other codes printed onto devices or packaging.
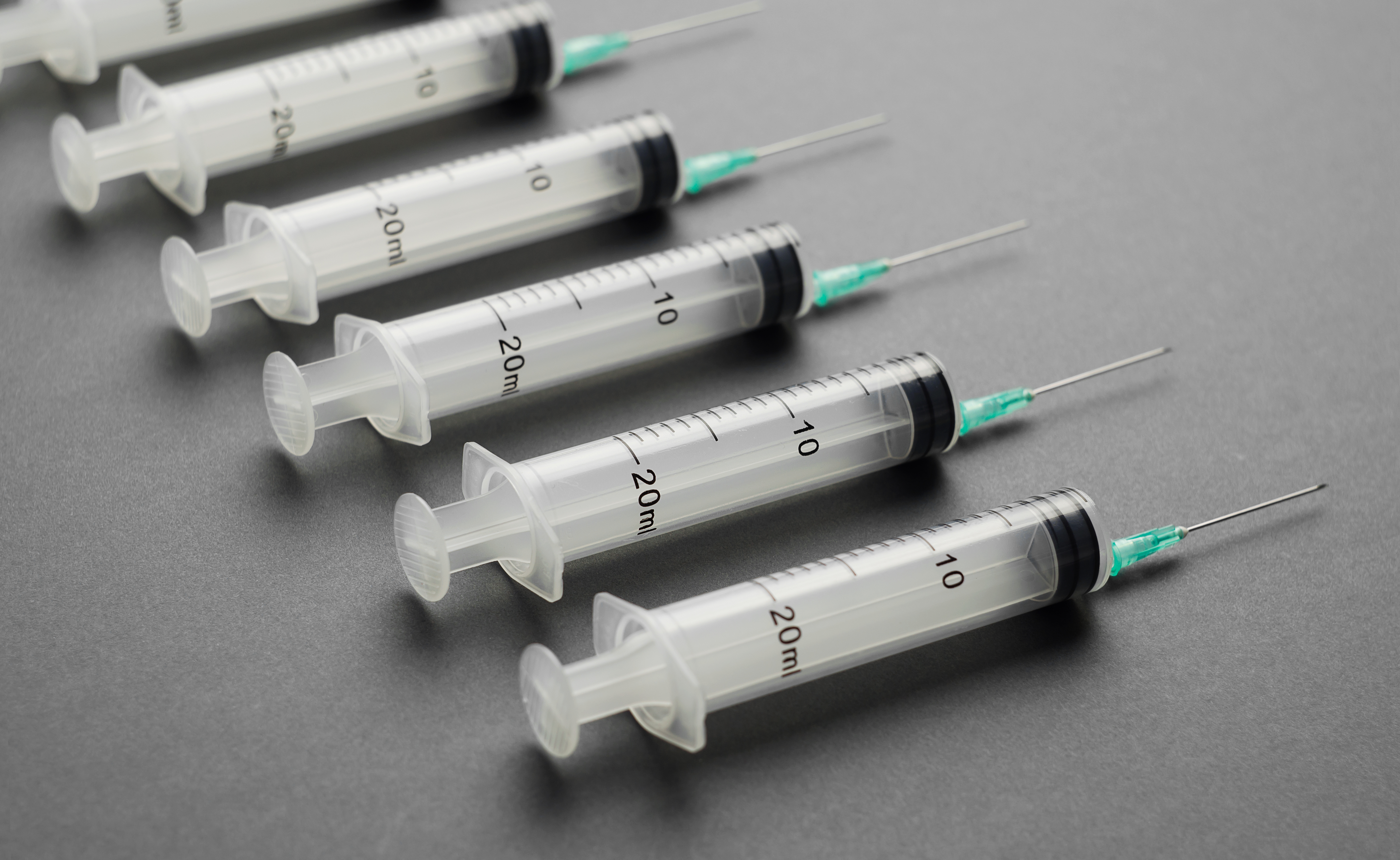
Most critical are markings essential to the use of the devices, such as measurement markings on catheters and syringes. Directions, warnings, usability guidelines and other identifying information must also be considered, both on the products and their packaging. Inks are applied during product assembly as well as during the packaging process, typically before instrument and package sterilization takes place.
Regardless of where and how they are applied, inks must meet certain performance characteristics.
First, the inks must have excellent adhesion. Not only do they need to be scratch resistant, they must also be impervious to heat, steam, alcohol, blood and other bodily fluids.
Although inks used on medical devices and their packaging are not supervised by the FDA, they must still meet regulatory requirements that prohibit the migration or “bleeding” of inks. There must be no risk of product contamination or loss of sterility. 
In addition, inks are frequently included in the sterilization of the product and its packaging. As such, they must be able to withstand the demands of standard techniques such as steam, ethylene oxide (EtO) and gamma radiation sterilization.
Ensuring safe, effective and sterile performance
 When selecting inks for medical devices and packaging, there are four critical supplier requirements medical device manufacturers and packaging firms must consider:
When selecting inks for medical devices and packaging, there are four critical supplier requirements medical device manufacturers and packaging firms must consider:
- Adhesion —More than any other type of inks, medical-grade formulas must stick to their devices or packaging and nothing else. Your provider should have the ability to formulate inks that consistently adhere to materials with specific surface tensions.
- Smart processes and controls —Your inks need to have the necessary resistance characteristics according to current good manufacturing practices (cGMP).
- FDA-compliant materials — When it comes to ensuring the safety of medical-grade inks, there’s no substitute for formulations composed exclusively of FDA-compliant raw materials. Although this strategy goes above and beyond the requirements for medical device inks, many companies consider it a worthwhile (if not essential) strategy for minimizing the potential for contamination. Since inks must pass biocompatibility and cytotoxicity testing, the use of FDA-compliant ingredients which consistently meet these testing standards is often viewed as a safer alternative.
- Quality management — Although many manufacturers can mix up a batch of ink capable of passing biocompatibility tests, these results may not be consistently repeatable because of the potential for cross-contamination within their facility. Colorcon’s facility is open to quality audits with all the proper procedures in place to ensure traceability and change control. Look for a partner that uses an ISO 9001:2015 registered quality management system when manufacturing medical device inks.
About Colorcon No-Tox® Products
Colorcon No-Tox® Products is a leading manufacturer of US FDA- and EU-compliant printing inks for all printing processes. All medical device inks comply with FDA 21 CFR, are manufactured in a cGMP, FDA and ISO 9001:2015 registered facility, and come with a written regulatory compliance guarantee.
No-Tox medical device inks are manufactured using raw materials classified by the United States Food and Drug Administration (FDA) as either direct or indirect food additives. Colorcon also offers contract manufacturing and tolling capabilities. Learn more.